
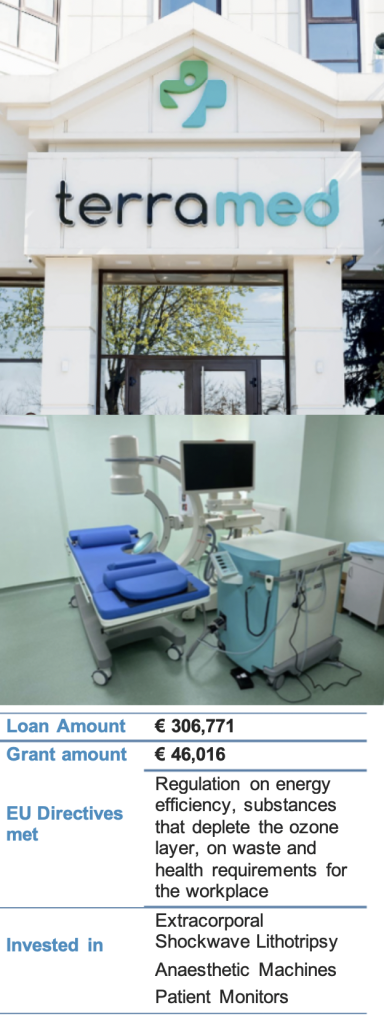
The company MedPharmConsult SRL has been providing medical services at the two sites of its Terramed clinic since 2011. The clinics have always provided high-level medical services with highly trained staff and modern equipment, offering a comprehensive range of medical and dental treatments for adults as well as children.
The company upgraded key equipment in one of the clinics to increase the intervention precision as well as to improve its occupational health and safety standards. The investment allows the company to offer medical services and working conditions compliant with local as well as EU standards. After the successful project verification the company received 15% of the loan value as a grant incentive, funded under the EU4Business initiative of the European Union.
With the investment, the company now meets a wide variety of European standards, including:
Directive 2014/68/EU on the harmonisation of the laws of the Member States relating to the making available on the market of pressure equipment (recast)
Developed and supported by:


Implemented by:

Verified by:
© Copyright 2021. All Rights Reserved.